Sustainable & Long-lasting: Natural Cosmetics Made from Purely Plant-based Ingredients
Should personal care products be sustainable or have a long shelf-life? Preferably both. Natural cosmetics are gaining in popularity due to the increased awareness of sustainability and naturalness. In contrast, there are potential quality limitations that discourage some consumers from buying certified natural cosmetic products. The use of natural oils and plant antioxidants could be the solution for both aspects. This could be beneficial for raw material and product manufacturing companies as well as for consumers. -> back to main page
Currently, there is a clear trend emerging in the cosmetics industry toward so-called “green cosmetics”. Natural cosmetics took 10.8% of the total cosmetics market in Germany in 2019, up from 6.9% in 2012. If nature-based cosmetics are also included here at 7.5%, green cosmetics reached a total share of 18.3% in 2019. This clearly shows that both natural cosmeticsand nature-based cosmetics are becoming increasingly important.Depending on the natural cosmetics label, there are differentrequirements as to which specific ingredients may be used. However, the use of synthetic oils and preservatives is largely excluded.In conventional cosmetics, standardized emollients are oftenused because they are more oxidatively stable than natural oils,which can contain traces and impurities that reduce shelf life. Inaddition, synthetic or chemically pure
antioxidants are also usedto protect against chemical spoilage due to oxidation, as they areoften more effective than natural antioxidant ingredients. Due to the exclusion of effective synthetic raw materials, especially incertified natural cosmetic products, the ingredients may oxidizethrough contact with oxygen and thus become rancid [1,2,3].
To understand how the process of oxidative quality change occurs, it is necessary to consider the composition of the lipophilic phase. This determines the product properties, such as the spreadability of an oil component on the skin. This determines, for instance, the richness and skin care feeling of a cream. To achieve a balanced skin feel, a mixture of oils, fats and waxes is used. The distribution of fatty acids is characteristic of the various fats and oils. Low, saturated fatty acids with up to four C atoms are liquid, more easily soluble in water and have a pungent odor. Medium fatty acids with five to ten C atoms are more oily, more difficult to dissolve in water and have a rancid odor. Higher fatty acids are more solid, insoluble in water and odorless. Unsaturated fatty acids have one or more double bonds and are more liquid the more double bonds a fatty acid has. Unsaturated fatty acids are preferred in cosmetics because they have advantages for the skin and improve the properties of the
emulsion. This makes it possible to control the consumer’s tactile perception or skin feel. Necessary ingredient claims can also be fulfilled by using special natural oils. Products with unsaturated fatty acids also absorb better into the skin. In terms of skin care properties, their use is therefore indispensable. A major problem with oils containing a high percentage of unsaturated fatty acids, however, is that they are oxidatively unstable and therefore have a shorter shelf life [4,5,6,7].
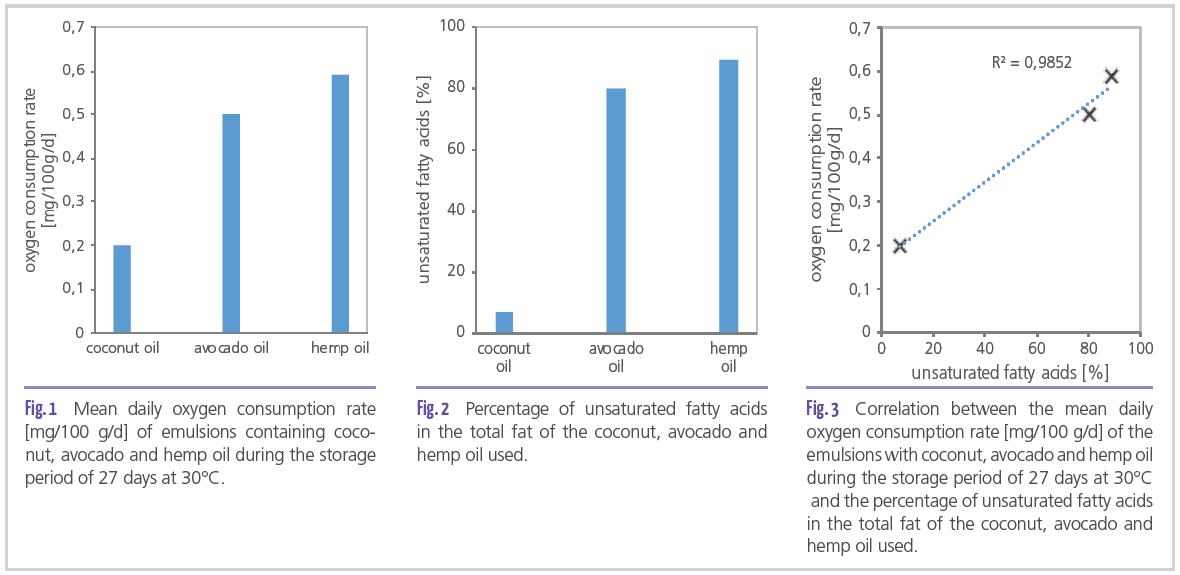
The extent of this shelf-life-reducing effect was investigated in the department “Retention of Food Quality” at the Fraunhofer Institute for Process Engineering and Packaging IVV. Specifically, the influence of different oils on the shelf life of creams was measured. Several O/W emulsions with the same formulation, pH value and manufacturing instructions were prepared with one type of oil, one emulsifier, one preservative and deionized water as a triple determination and stored. Meanwhile, the oxygen concentration in the head space of the vial was measured. From the reduction of oxygen concentration, the oxygen consumption rate was calculated, that is, the daily oxygen intake per 100 g of product. Three oils were selected: Coconut oil with a high content of saturated fatty acids, avocado oil with a high content of monounsaturated fatty acids and hemp oil with a high content of polyunsaturated fatty acids. The emulsion showed no signs of instability during storage.
In the following figures (Figures 1-3), it is clearly visible that the oxygen consumption rate correlated with the proportion of unsaturated fatty acids in the total fat content of the respective oil. The higher the proportion of unsaturated fatty acids, the higher was also the daily oxygen uptake.
From these results it can be concluded that oxidative spoilage accelerated the more unsaturated fatty acids were present in the emulsion. High oxygen uptake leads to the formation of rancid fat oxidation products and a possible rejection by consumers due to off-odor. Thus, it is clear that the use of oils with unsaturated fatty acids requires additional ingredients to compensate for the reduction in shelf life. Antioxidants can be used for this purpose, but they often have high raw material prices and are used in large quantities. This results in an enormous savings potential if these raw materials are replaced by cheaper and more effective alternatives.
To protect sensitive oils and emulsions from oxidation, plant extracts can be used in addition to classical antioxidants. Plants produce tens of thousands of different secondary plant substances (SPS) that perform various ecological functions, such as protection against herbivores, pests, pathogens and UV radiation [8,9,10]. They are known for their strong antioxidant effect, whereby they can scavenge reactive oxygen species and other free radicals. Synergistic combination of different SPS can not only increase the antioxidant effect, but also additionally improve other functionalities, such as viscosity and spreadability [11,12,13,14,15].
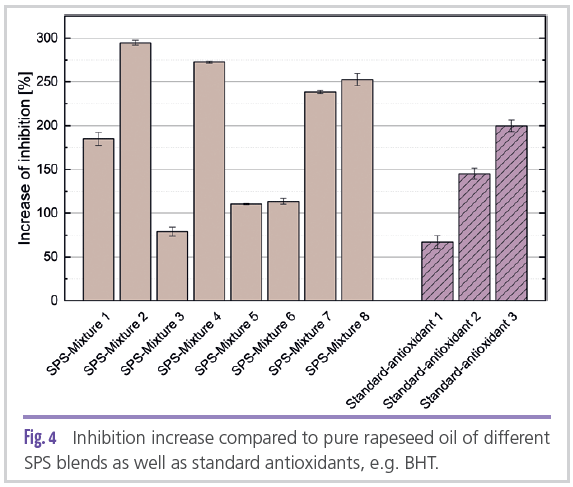
In the department “Process Development for Plant Raw Materials” at the Fraunhofer IVV, efficient extraction processes are developed for the isolation of secondary plant compounds from various raw and residual plant materials. During extraction, parameters such as solid-liquid ratio, extraction time and temperature as well as the type of solvent are varied in order to optimally adapt the extracts to their target application. By adding different enzymes and using suitable stirring and reactor systems, the yield of functional substances in the extract can be further increased. We also develop model formulations based on water, solvents or oil to incorporate the extracts into different liquid media. Depending on the application, it is possible to produce emulsions or solutions in which the substances can act in the solution itself or at the interfaces [16,17,18]. In the following, the results (see Figure 4) of various SPS mixtures in rapeseed oil from Rancimat measurements are shown as an example in comparison with three standard antioxidants. Both the extracts (with solubilizer) and the standard additives were added at concentrations of 1 wt.%.
The results show that an increase in oxidation stability was achieved by both the single extracts and the
mixtures. Accordingly, the oxidation stability of the oil could be increased by about 1.5 times by adding the plant extracts compared to the standard additives used. Thus, it could be shown that biobased antioxidants from plant extracts have the potential in many application areas to slow down oxidative changes and thus extend shelf life.
Although valuable unsaturated fatty acids increase the oxidation of emulsions, the targeted use of antioxidants from plant extracts can effectively prevent this oxidation. This can improve the quality of the product while saving raw materials and costs, making the overall product concept more sustainable and long-lasting. We can support you with our established methods in the investigation of your products and the selection of the right plant extracts for your cosmetic applications.

Read more about the use of secondary plant compounds.
Contact us:
Tel.: +49 8161 491 470
Mobile: +49 1716 411 383
[email protected]
www.ivv.fraunhofer.de
Dipl.-Leb.Chem Arielle Springer
studied food chemistry at the TU Dresden and gained professional experience as a product developer in cosmetics. Together with experienced experts, she is currently working as a Business Development Manager interdisciplinary on the continuous development of the research field Personal & Home Care.

Authors
A. Springer*, M. Platzer, Dr. S. Kiese | Fraunhofer Institute for Process Engineering and Packaging IVV | Giggenhauser Str. 35 | 85354 Freising | Germany
E. Destler, B. Pazurik | Hochschule Weihenstephan-Triesdorf, Fakultät Gartenbau und Lebensmitteltechnologie | Am Staudengarten 10, | 85354 Freising | Germany
*Corresponding Author
References
[1] Statista: Marktanteile von Naturkosmetik bis 2019. URL: https://de.statista.com/statistik/daten/studie/155668/umfrage/marktanteile-von-naturkosmetik- im-deutschen-kosmetikmarkt/, Zugriff am 16.12.2020
[2] Heinze, K. (2019): Naturkosmetik im Trend. KOSMETIK international, 4, S. 18–22.
[3] Schreier, S.: Die wichtigsten Naturkosmetik-Siegel im Überblick - BDIH, Natrue, Demeter, Ecocert. URL: https://www.welt.de/icon/beauty/article175839873/ Die-wichtigsten-Naturkosmetik-Siegel-im-Ueberblick-BDIH-Natrue-Demeter-Ecocert. html, Zugriff am 16.12.2020.
[4] Umbach, W. (2004): Kosmetik und Hygiene von Kopf bis Fuß. Wiley-VCH, Weinheim,3. Aufl.
[5] Bährle-Rapp, M. (2004): Springer Lexikon Kosmetik und Körperpflege. Springer Verlag, Berlin, 2. Aufl.
[6] Belitz, H.-D.; Grosch, W.; Schieberle, P. (2008): Lehrbuch der Lebensmittelchemie.Springer Verlag, Berlin, 6. Aufl.
[7] Schubert, H.; Armbruster, H. (1989): Prinzipien der Herstellung und Stabilität von Emulsionen. Chemie Ingenieur Technik, 61, S. 701–711.
[8] Shahidi, F.; Naczk, M. (1995): Food phenolic: sources chemistry effects applications. Lancaster PA, USA: Technomic Publishing Company Co..
[9] Bennett, R.; Wallsgrove, R. (1994). Sesondary metabolites in plant defence mechanism. New Phytol., Bd. 127, Nr. 4, pp. 617-633.
[10] Crozier, A.; Clifford, M.; Ashihara, H. (2008): Plant Metabolies: Occurence, Structure and Role in the Human Diet. NJ, USA: John Wiley & Sons.
[11] Shahidi, F.; Zhong, H.; Ambigaipalan, P. (2005): Antioxidants: regulatory status. Bailey’s Ind. Oil Fat Prod, Bd. 1, Nr. 1, pp. 1-21.
[12] Zheng, W.; Wang, S. (2001): Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem., Bd. 49, Nr. 11, pp. 5165-5170.
[13] Dragland, S.; Senoo, H.; Wake, K.; Blomhoff, R. (2003): Several culinary and medicinal herbs are important sources of dietary antioxidants. J. Nutr., Bd. 133, Nr. 5, pp. 1286-1290.
[14] Wang, S. (2003): Antioxidant Capacity of Berry Crops and Herbs. Oriental Foods and Herbs, Wachington DC, USA, American Society, ACS Symposium Series Vol 859, pp. 190-201.
[15] Wu, X.; Beecher, G.; Holfe, J.; Haytowitz, D.; Gebhardt, S.; Prior, R. (2004): Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J. Agric. Food Chem., Bd. 52, Nr. 12, pp. 4026-4037.
[16] Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Eisner, P. (2021): How Does the Phenol Structure Influence the Results of the Folin-Ciocalteu Assay? Antioxidants, 10, 811.
[17] Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Miesbauer, O.; Eisner, P. (2021): Common Trends and Differences in Antioxidant Activity Analysis of Phenolic Substances Using Single Electron Transfer Based Assays. Molecules, 26, 1244.
[18] Shahidi, F.; Janitha, P.; Wanasundara, P. (1992): Phenolic antioxidants. Crit. Rev. Food Sci. Nutr., 32, 67–103






