Boosting Cellular Recycling System for a Positive Skin Aging
Face and hands are the first body regions to show signs of aging [1,2]. One of its manifestations is the appearance of age spots, which are the result of lipofuscin, oxidized or damaged proteins, and other cellular residues in the lysosomes [3,4,5,6].
The recovery mechanism for damaged macromolecules and proteins is controlled by both the autophagic process and proteasome activity [7].
This study is intended to evaluate the potential of a plant extract composed of Myrothamnus flabellifolia and Coffea arabica (MCE) to reduce the appearance age spots through autophagy and proteasome activity. In vitro studies in human fibroblasts with MCE showed a decrease in mTOR gene expression and an increase in Parkin, in addition to stimulating proteasome activity. These results are corroborated by a 56-day clinical evaluation, focusing on the face and hands, that indicated MCE lightens age spots and evens skin tone.
MCE mitigates the signs of aging by promoting homeostasis of the cellular detoxification system through stimulation of autophagy and proteasome activity that eliminates toxins and dysfunctional cellular biological components. -> back to main page
Introduction
Skin aging is a complex process involving both biological and molecular changes. Several phenotypes found in aged skin tissue are triggered by the presence of damaged molecules and organelles in the cells. Studies indicate that their proliferation is controlled by the autophagy process, which can result from three distinct mechanisms: microautophagy, chaperone-mediated autophagy (CMA), and macroautophagy. Of the three mechanisms mentioned, macroautophagy, through the lysosomes that inhabit human cells, is the most studied mechanism [8].
One of the responses to cellular aging is the accumulation of lipofuscin protein aggregates, lysosomes with excess cellular residues, due to reduced cellular recycling efficiency. Lipofuscin is a yellowish-brown pigment found in many cells of the body, such as keratinocytes, fibroblasts, neurons, myocytes, and hepatocytes. Such accumulation in the skin results in the appearance of age spots, different from each other in shape, size, color, and degree of projection. They are most frequently found on the body where the skin has greatest exposure to the sun, such as the face or back of the hands [9,10].
The homogeneity of skin tone, depending on the age of the individual, is also threatened by the accumulation of carbonyl proteins, which are generated by the attack of reactive oxygen species in the skin tissue [11].
Carbonylated proteins are products of the reaction between amino groups and reactive aldehyde compounds produced from lipid peroxidation, which is initiated by reactive oxygen species. Thus, in the skin, carbonyl proteins are more frequently detected in places exposed to the sun in the elderly, which contributes to the change in skin color to dark yellow due to extrinsic aging [12]. Carbonyl proteins also impact the proteasome system, as low-molecular-weight carbonyl proteins are degraded by proteasome activity. However, high-molecular-weight proteins are resistant to degradation and, therefore, inhibit the action of proteasomes [13]. The proteasome is a sophisticated and extremely efficient protein complex that acts through selective hydrolysis along with the ubiquitin molecule in signaling the degradation of numerous target proteins. Just like the autophagic process, proteasome activity catalyzes biological reactions for the digestion of damaged or malformed proteins [14].
The search for stimulators of recycling systems involves the study of numerous compounds, including plant extracts due to their rich chemical composition which, naturally, entails a wide possibility of biological functions. In this sense, our research group deepened its studies on the extract of Myrothamnus flabellifolia, the “resurrection plant” well known in traditional African medicine due to its ability to survive extreme dehydration for many years, as well as on the extract of coffee (Coffea arabica), an important source of antioxidant in the human diet, obtained from upcycling coffee seedcake. The phytochemical profile of the Myrothamnus flabellifolius
leaf extract is composed of flavonoids, anthocyanins, alkaloids, steroids, terpenoids, triterpenes, cardiac glycosides, saponins, phlobatanins, tannins, polyphenols, and sugars, including trehalose, a compound that became widely known for its ability to induce cellular autophagy and mitigate diseases related to protein aggregation [15].Coffee extract, on the other hand, is rich in phenolic compounds and their derivatives (such as chlorogenic acids), alkaloids (especially caffeine), diterpenoid alcohols, carbohydrates, lipids, and volatile and heterocyclic compounds [16]. Lipofuscin is a peroxidized lipid-protein aggregate that accumulates during aging or under conditions of oxidative stress that can occur as a result of decreased antioxidant defenses or loss of repair functions. In other words, the presence of antioxidants is essential in protecting against oxidative stress [17].
Materials & Methods
Expression of mTOR and Parkin genes associated with Autophagy
Human, neonatal dermal fibroblast cultures (HDFn C-004- 5C, Cascade Biologics™, CA, USA) were incubated with 0.313 mg/mL (non-cytotoxic concentration) of MCE for 24 hours. Cell lysates were collected and submitted to RNA extraction using Purelink™ (Life Technologies) and quantified by spectrometry using NanoDrop Lite (Thermo Fisher Scientific), followed by RT-PCR analysis in StepOnePlus equipment (Applied Biosystems). Gene expression was performed using commercial kits (TaqManR Assays Gene Expression) for the following genes: mTOR (Mechanistic Target of Rapamycin Kinase) Hs00234508_m1; PARK2 (Parkinson protein 2) Hs01038322_m1 (Applied Biosystems). The test was performed using TaqManR RNA-to-CT™ 1-Step Kit (Applied Biosystems), under experimental conditions of 48°C for 30 minutes for reverse transcription, 95°C for 10 minutes for activation of AmpliTaq GoldR DNA Polymerase, followed by 50 cycles at 94°C for 15 seconds and at 60°C for 1 minute for denaturation and rewarming, respectively. The relative amount of mRNA was calculated by a mathematical model for relative quantification and real-time gene expression analysis and by the 2−ÄÄCT method.
Ubiquitin-Proteasome System Activity
Normal human dermal fibroblasts (NHDF) were cultured in NHDF culture medium (Sigma-Aldrich/Merck KGaA) overnight. Then, the culture medium was replaced by a fresh culture medium containing betulinic acid at 20 μg/ml (positive control) and MCE at 0.0375 mg/ml. An untreated cell group was cultured in a regular growth medium and incubated under the same experimental conditions (control group). Cell viability was previously verified by staining with Trypan Blue solution (Bio-Rad Laboratories Inc). After 24 h of incubation with each product, chymotrypsin-like proteasome activity was measured kinetically in all samples for 60 minutes. Measurements were taken every 2.5 min. The luminescence generated from proteasome cleavage of a peptide substrate added to the samples was quantified using Proteasome-Glo™ chymotrypsin- like cell-based assay kit (Promega Corp).
Lipofuscin Expression
Lipofuscin expression was performed by immunofluorescence in human, neonatal dermal fibroblast cultures (HDFn C-004- 5C, Cascade Biologics™, CA, USA) using antibody-enhanced detection of senescent cell reagent (SenTraGor™, Arriani Pharmaceuticals, Attica, Greece) and a Leica-DM 6000B fluorescence optical microscope coupled with a 2.8 MP Leica DFC7000T camera. Images were captured using LAS software (Leica Application Package v.4.12) and qualitatively evaluated by the intensity of fluorescence emitted by labeling with specific antibodies.
Clinical Trial
A randomized, single-blind, placebo-controlled clinical trial was performed with 34 subjects for 56 days. One group used a cream containing 2% w/w of MCE and the other group used a placebo, applying the solution to the face and hands twice a day. Age spots on the hand were evaluated through the analysis of standardized images captured with a Canon EOS 60D Digital Camera. Facial spots were evaluated using images captured with Visia CR equipment (Canfield Scientific, Inc.). The captured images were used to evaluate skin uniformity and luminosity, comparing the images taken before and after product use. A specific software program was used to analyze the images and the ‘total surface of spots’ and ‘Haralick Homogeneity Index’ parameters were used.
Results and Discussion
mTor and Parkin are two important proteins involved in the autophagic process via macroautophagy (Figure 1) [18].
mTOR forms the mTORC1 and mTORC2 complexes, the former being responsible for modulating the autophagy process [19]. During nutrient deprivation, mTORC1 separates from the protein complex (ATG13 and ULK1/2), which causes their partial dephosphorylation, and
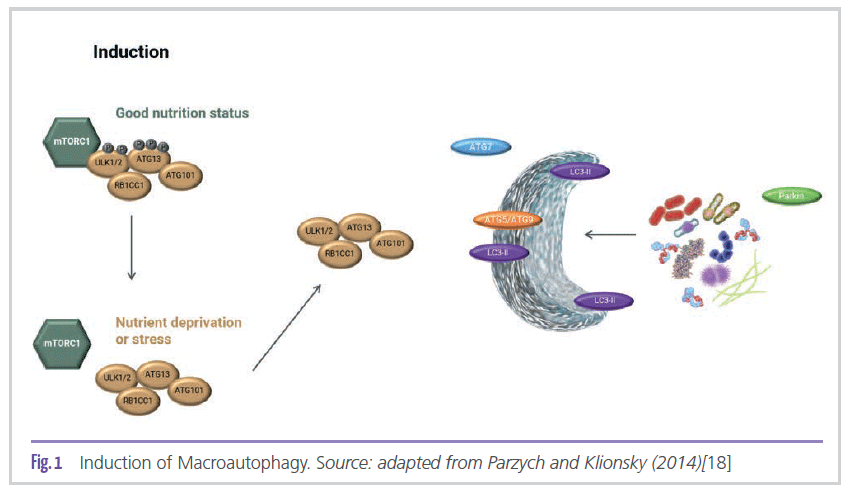
as a result, autophagy is induced. Jung et al. (2010) state that among the numerous proteins that regulate autophagy, mTOR (target of rapamycin) is the key component that maintains homeostasis between the autophagic process in response to the cell’s physiological conditions and environmental stress [20]. In other words, the absence of mTOR is key for the induction of cellular autophagy. As a result of autophagy induction, ATG13 and ULK1/2 proteins play a role in the biogenesis of phagophore and autophagosome [21], in addition to RB1CC1, a protein found in the initial and later events of autophagosome formation [22]. LC3 is a key protein in the regulation of autophagy and autophagosome biogenesis after becoming membrane-conjugated through interactions with ATG7 and other autophagic effectors [23]. At the same time, ATG9 plays a fundamental role in organizing the structure of the phagophore. In addition to the proteins involved in the formation of the autophagosome, it is also important to detect proteins that tag the compounds to be degraded. According to Narendra et al. (2008), PARK2, also known as the Parkin protein, is responsible for monitoring and selecting damaged mitochondria, in addition to mediating the process of sending them to autophagosomes and their subsequent degradation [24].
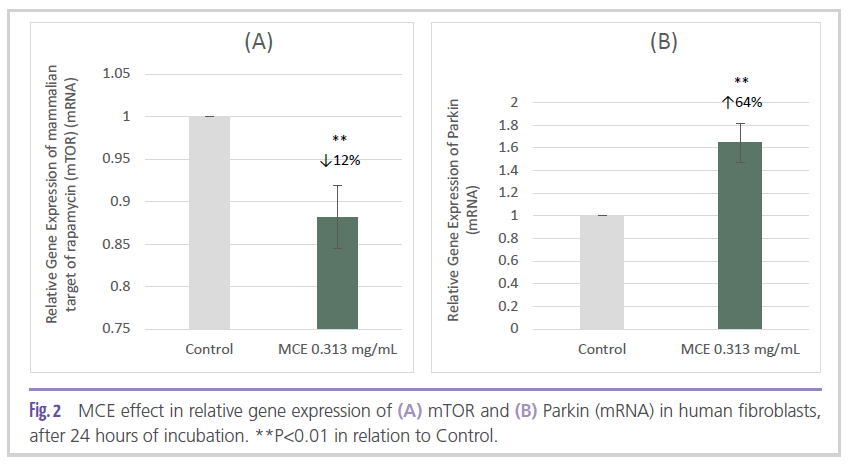
In vitro studies carried out in a human fibroblasts culture indicated the potential of MCE to modulate two of the main proteins associated with the autophagic process. The ingredient applied at 0.313 mg/mL significantly reduced mTOR gene expression by 12% (Figure 2A) and significantly increased Parkin gene expression by 64% (Figure 2B) when compared to control.
The action of autophagy alone is not enough to prevent the formation of lipofuscin [25]. On the other hand, it is known that the proteasome
system is responsible for decreasing the formation of this pigment. Nevertheless, the accumulation of lipofuscin also inhibits proteasome activity [26]. In this sense, autophagy is the most efficient way to remove oxidized proteins and inhibit the formation of aggregates in aged cells due to reduced proteasome activity efficiency [27], since the processes also show differences depending on the age of the individual. Recent studies carried out with human fibroblasts indicate that genetic markers associated with the autophagic flux show virtually no differences between young and elderly individuals. However, the increased production and accumulation of damaged proteins due to aging alters the homeostasis of cell recycling [28]. In contrast, proteasome activity and content are downregulated with the increasing age of the individual. Thus, unlike the autophagic flux, proteasome activity decreases throughout life, and
consequently there is less degradation of oxidized proteins and an increase in protein aggregation, which induces cellular degeneration and several diseases associated with aging [29]. That said, it is only possible to mitigate excessive “cellular waste” with high levels and effectiveness of these two systems: autophagy and proteasome.
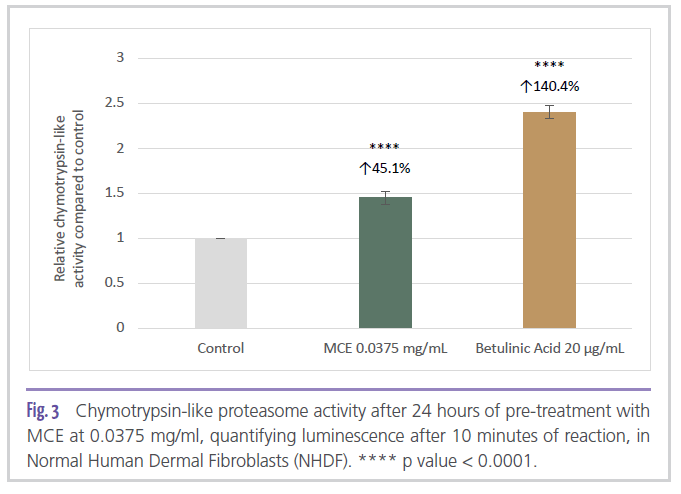
In addition to modulating the gene expression of autophagy-related markers, in vitro treatment of Normal Human Dermal Fibroblasts (NHDF) with 0.0375 mg/ml of MCE for 24 hours indicated that the ingredient was able to significantly stimulate proteasome activity (chymotrypsin-like proteasome activity) by 45% compared to the untreated control (Figure 3).
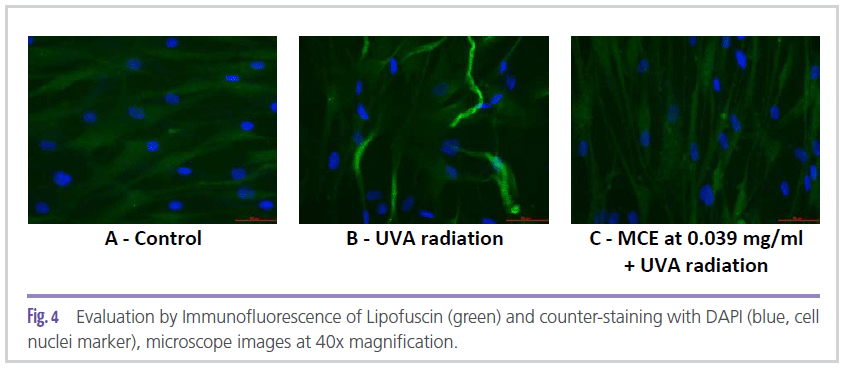
The role of MCE via autophagy and proteasome activity was confirmed through lipofuscin expression evaluation in fibroblast culture. As can be seen in Figure 4C, MCE at 0.039 mg/ mL was able to minimize the synthesis of lipofuscin generated by exposure to UVA radiation (Figure 4B), matching the control group without radiation stress (Figure 4A).
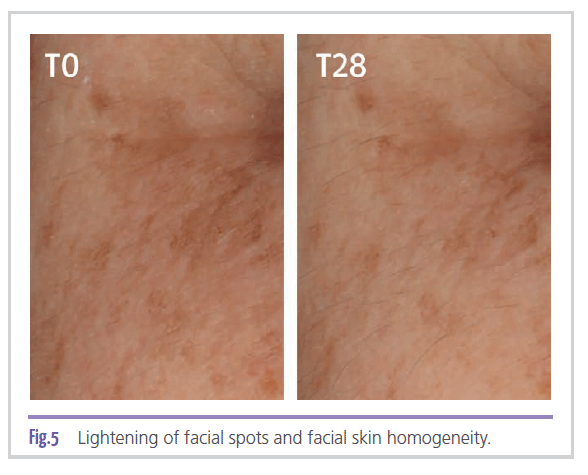
In addition to the in vitro effects verified on the autophagy system, represented herein by the modulation of two of its most relevant biological markers: mTOR and Parkin, and on the proteasome system and its subsequent role in the synthesis of lipofuscin, a clinical trial was also conducted to measure the actual effectiveness of the ingredient in treating age spots. In Figure 5,
lightening of facial spots and improved skin tone homogeneity are observed in a subject who used a cream containing 2% MCE for 28 days. The results obtained through image capture with Visia CRR equipment showed a significant 42%
improvement (p < 0.001) in facial skin tone homogeneity after 28 days, when compared to baseline. Placebo showed an 11% improvement (p < 0.05). As for spot size, it was observed a 66% (p < 0.001) improvement with the use of the ingredient when compared to baseline. In the case of spots on the hands, the improvement is more significant after 56 days, being 63% (p < 0.05) for the ingredient compared to baseline. The placebo showed no significant improvement. As for spot size, there was an improvement of 26% (p < 0.05) compared to baseline. The placebo showed no significant improvement.
Conclusion
Although autophagy and proteasome activity are distinct biological processes, acting on both mechanisms to increase the recycling capacity of proteins in the cytosol of cells proves to be an effective and synergistic strategy to reduce the signs of skin aging, since both metabolic pathways are specialized in degrading their respective targets. Therefore, the homeostasis of the system must result from the communication of these mechanisms in a compensatory and effective way. In this sense, the ingredient shown here, commercially known as Iselight, can act on the root cause of age spot formation, promoting homeostasis of the cellular, autophagic, and proteasome detoxification systems that eliminate toxins and dysfunctional biological components in the cell.
Authors
Lilian Mussi, MSc.* | [email protected]
Innovation Manager – Research and Development Department
Rafael Carlos Bíscaro |
[email protected]
Skin Care Marketing Specialist – Product Marketing
Wagner Vidal Magalhães |
[email protected]
Scientific Director – Research and Development Department
Chemyunion Ltda | Sorocaba | Brazil
*Corresponding Author
References
[1] GOLD, Michael H.; GALLAGHER, Conor. An evaluation of the benefits of a topical treatment in the improvement of photodamaged hands with age spots, freckles, and/or discolorations. Journal of Drugs in Dermatology: JDD, v. 12, n. 12, p. 1468-1472, 2013.
[2] FARAGE, Miranda A. et al. Structural characteristics of the aging skin: a review. Cutaneous and Ocular Toxicology, v. 26, n. 4, p. 343-357, 2007.
[3] RINNERTHALER, Mark et al. Oxidative stress in aging human skin. Biomolecules, v. 5, n. 2, p. 545-589, 2015.
[4] YAMAGUCHI, Yuji; BRENNER, Michaela; HEARING, Vincent J. The regulation of skin pigmentation. Journal of Biological Chemistry, v. 282, n. 38, p. 27557-27561, 2007.
[5] PORTA, Eduardo A. Pigments in aging: an overview. Annals of the New York Academy of Sciences, v. 959, n. 1, p. 57-65, 2002.
[6] SKOCZYŃSKA, Anna et al. Melanin and lipofuscin as hallmarks of skin aging. Advances in Dermatology and Allergology/ Postępy Dermatologii i Alergologii, v. 34, n. 2, p. 97, 2017.
[7] TANAKA, Keiji. The proteasome: overview of structure and functions. Proceedings of the Japan Academy, Series B, v. 85, n. 1, p. 12-36, 2009.
[8] GIORGIO, Selma. Autofagia celular em processos patologicos. Semina: Ciencias Biologicas e da Saude, v. 35, n. 1, p. 125-136, 2014.
[9] WANG-MICHELITSCH, Jicun; MICHELITSCH, Thomas M. Development of age spots as a result of accumulation of aged cells in aged skin. arXiv preprint arXiv: 1505.07012, 2015.
[10] KÖNIG, Jeannette et al. Mitochondrial contribution to lipofuscin formation. Redox biology, v. 11, p. 673-681, 2017.
[11] OLIVER, Cynthia N. et al. Age-related changes in oxidized proteins. Journal of Biological Chemistry, v. 262, n. 12, p. 5488-5491, 1987.
[12] YAMAWAKI, Yumiko et al. The impact of carbonylated proteins on the skin and potential agents to block their effects. Experimental dermatology, v. 28, p. 32-37, 2019.
[13] GONOS, Efstathios S. et al. Origin and pathophysiology of protein carbonylation, nitration and chlorination in age-related brain diseases and aging. Aging (Albany NY), v. 10, n. 5, p. 868, 2018.
[14] TANAKA, Keiji. The proteasome: overview of structure and functions. Proceedings of the Japan Academy, Series B, v. 85, n. 1, p. 12-36, 2009.
[15] DEBOSCH, Brian J. et al. Trehalose inhibits solute carrier 2A (SLC2A) proteins to induce autophagy and prevent hepatic steatosis. Science signaling, v. 9, n. 416, p. ra21-ra21, 2016.
[16] AFFONSO, Regina Celis Lopes et al. Phytochemical composition, antioxidant activity, and the effect of the aqueous extract of coffee (Coffea arabica L.) bean residual press cake on the skin wound healing. Oxidative medicine and cellular longevity, v. 2016, 2016.
[17] AMES, Bruce N.; SHIGENAGA, Mark K.; HAGEN, Tory M. Oxidants, antioxidants, and the degenerative diseases of aging. Proceedings of the National Academy of Sciences, v. 90, n. 17, p. 7915-7922, 1993.
[18] PARZYCH, Katherine R.; KLIONSKY, Daniel J. An overview of autophagy: morphology, mechanism, and regulation. Antioxidants & redox signaling, v. 20, n. 3, p. 460-473, 2014.
[19] Kim YC, Guan KL. mTOR: a pharmacological target for autophagy regulation. J Clin Invest. 2015;125:25-32.
[20] JUNG, Chang Hwa et al. mTOR regulation of autophagy. FEBS letters, v. 584, n. 7, p. 1287-1295, 2010.
[21] GRASSO, Daniel Hector; RENNA, Felipe Javier; VACCARO, Maria Ines. Initial Steps in Mammalian Autophagosome Biogenesis. Frontiers in cell and developmental biology, v. 6, p. 146, 2018.
[22] SZKLARCZYK D, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019 Jan; 47:D607-613
[23] HUANG, Rui; LIU, Wei. Identifying an essential role of nuclear LC3 for autophagy. Autophagy, v. 11, n. 5, p. 852-853, 2015.
[24] NARENDRA, Derek et al. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. The Journal of cell biology, v. 183, n. 5, p. 795-803, 2008.
[25] KELLER, Jeffrey N. et al. Autophagy, proteasomes, lipofuscin, and oxidative stress in the aging brain. The international journal of biochemistry & cell biology, v. 36, n. 12, p. 2376-2391, 2004.
[26] HÖHN, Annika et al. Lipofuscin inhibits the proteasome by binding to surface motifs. Free Radical Biology and Medicine, v. 50, n. 5, p. 585-591, 2011.
[27] RINNERTHALER, Mark et al. Oxidative stress in aging human skin. Biomolecules, v. 5, n. 2, p. 545-589, 2015.
[28] KIM, Hei et al. Autophagy in human skin fibroblasts: Impact of Age. International journal of molecular sciences, v. 19, n. 8, p. 2254, 2018.
[29] BULTEAU, A.-L.; PETROPOULOS, I.; FRIGUET, B. Age-related alterations of proteasome structure and function in aging epidermis. Experimental gerontology, v. 35, n. 6-7, p. 767-777, 2000.






