Is My Sun Protection Fading Away?
The Impact of Photostability on Beach Goers, SPF Claims and the Environment
Sunscreens are widely used to protect against damaging effects of sun exposure. Avoiding the pain of sunburn is a key motivator for applying sunscreen, however, these products offer much broader protection because when applied correctly and balanced with informed and managed exposure, they can also protect against skin cancer and minimize skin ageing effects. A product category that has such widespread use needs to be very safe in all foreseeable ways. It also needs to be compatible with the environment, in particular marine flora and fauna as it will often be used in coastal areas such as the beach. Due to the general popularity of sunscreens, concerns of any kind tend to spread rapidly, even if an adversity claim has only been formulated as a potential hazard without proper investigation or evaluation of the likelihood and impact of the risk. Even for scientists, risk evaluations are labor-intensive processes that require a solid scientific education, so unsurprisingly, consumers who cannot make such evaluations on their own, often adopt an avoidance strategy towards potential hazards. In the case of sunscreens though, this increases the risk further, and not just in terms of sunburn, because the skin cancer incidence rate also increases. In view of this, it is of utmost importance to stick to the facts, to perform a risk analysis on hazard claims, and to provide clear information to consumers before they consider avoidance actions which may entail not so obvious downsides.
Another concern that has materialized around sunscreen centers on the fact that some UV filters – or combinations of UV filters – are not photostable and degrade, leading to a loss of performance. It sounds like a contradiction in terms that sun radiation itself could reduce the efficacy of a product designed to protect against such radiation. We have therefore revisited this topic and investigated state of the art formulas to assess how much performance is lost after a full day at the beach or on a sunny winter’s day skiing outdoors. We have also looked at the impact of photostability and claim making. What strategy should be followed – especially when creating high protection formulas? Finally, we have considered what photo-stability could mean in terms of the environment. -> back to main page
What is photo-instability
Molecules that absorb light are transferred into excited states. The energy absorbed can be lost through several mechanisms with different rate constants, ranging from extremely short to minutes and hours. UV light contains considerable energy, enough to potentially trigger bond breakage, configurational molecular changes, and new molecule formations. Generally, there are four possible types of reaction that do not result in the molecule returning to its relaxed ground state and could also destroy the molecule’s absorptive properties:
1. Photo-destruction
Compound A -> Break down compounds with no UV absorption
Example: Avobenzone, which at a certain yield can enter the long-lived triplet stage, from which bond breakage is possible which then leads to smaller non-absorbent compounds.
2. Photo-isomerization
Compound B1 -> Compound B2 (Isomer)
Examples: Octyl methoxycinnamate (OMC) in its excited state has a very much weakened double bond which in turn leads to isomerization forming a larger proportion of the Z-configurated isomer. This reaction is reversible and leads quickly to an equilibrium mixture of E- and Z-
isomer. Note that this does not destroy the chromophore, however, the Z-isomer has a lower extinction. Performance thus stabilizes at the equilibrium mix. Additionally, Avobenzone undergoes isomerization from the enol to keto-form. The keto-form only has some UVB absorption properties and no UVA absorption, however, protonation-deprotonation reactions quickly regenerate the enol back, which is why, generally, this process cannot be observed within sunscreens using regular spectroscopic methodology. Nevertheless, working with diluted HPLC samples in solvents requires attention in sunscreen analysis and the samples need to be protected from daylight.
3. Photo-reactions
Compound A + Compound B -> Adduct with no UV absorption
Examples: different reactions are possible here. An important type of reaction is the [2+2] cycloaddition, where two molecules with a double bond fuse together to form a cyclobutane derivative. That is not very stable in light and reacts further to other products that all show no effective absorption. Molecular partners that are well known for this reaction type are OMC+OMC and Avobenzone + OMC. There is currently no technology to prevent [2+2] cycloaddition. Furthermore, the combination of Diethylamino hydroxybenzoyl hexyl benzoate (DHHB) and Avobenzone is not photostable and leads to the destruction of both compounds [1].
4. Oxidative processes
Oxidative processes can also occur, but they require the presence of oxygen and enabling chromophores for activation, e.g.: by formation of singlet oxygen. Sunscreen chromophores in general should not be good activators at all, because potent activators belong to the category of photo-toxic compounds and would not make it through registration. Nevertheless, with very low quantum yields this process might happen in sunscreen, also triggered by other materials. Therefore, adding small amounts of anti-oxidants to sunscreens is advised.
The “photo-destruction” mechanism plays a pivotal role in sunscreen photostability. This is easy to characterizethrough laboratory experiments on templates covered with sunscreens as it is a feature of a single compound. HPLC supported methods are also available to quantify loss of material, e.g. [2]. Most concerns centered around this topic therefore arise when discussing sunscreen photostability, particularly in relation to Avobenzone.
The impact of photostability on consumers using sunscreens
To approach this question, it is important to understand what kind of radiation burden consumers are exposed to. This seems to be an easy question and easy to measure with photometric devices. However, collecting the exposure dose human skin receives is rather tricky. Solar light intensity depends on so many factors, e.g.: global location, calendar day, cloud cover, dust particles in the air, time of day, and the angle at which the sun hits the skin’s surface [3] and reflective objects like snow or water, adding further burden to direct sun exposure. For these reasons, measuring sun intensity with a fixed device somewhere, or sporadically measuring sun intensity, are not accurate approaches. The dosimeter needs to be worn by a subject all day long. Fortunately, a couple of studies have already dealt with this topic under realistic and intense exposure conditions. Test subjects have been sent on beach holidays in the Canary Islands and Egypt or on skiing holidays in Austria [4,5] wearing such dosimeters. Outdoor workers who are exposed to intensive periods of sunlight in their professional routines have also been followed with wrist dosimeters [6,7]. The total energy of sun light to skin was not the focus in these studies, only the erythemal energy was registered, utilizing the sensitivity function of human skin towards erythema (e.g.: ISO/CIE 17166:2019). That way, dosages could be compared with daylight radiation devices used in laboratories to test sunscreens for photo-stability and the solar simulated radiation (UVA and UVB light 10:1) used to test subjects when measuring and testing Sun
Protection Factor (SPF).
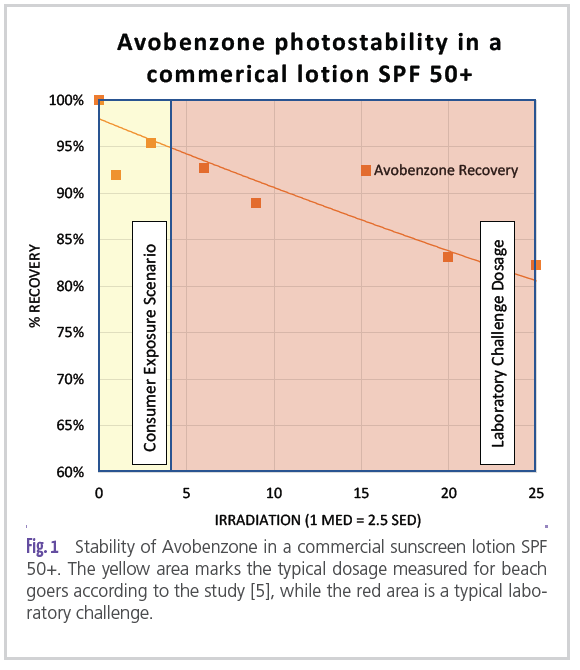
The unit of measurement proposed to measure this dosage is the Standard Erythemal Dose (SED) [8,9]. In comparison to the Minimal Erythemal Dose (MED), the SED is not skin type dependent and allows easy and well-defined comparison of light sources towards erythemal action. One SED is set to 100 J/m2. For example, a skin type 2 person would require between 2.4 and 4 SED to receive an erythema (1 MED). According to a study by Peterson [4], beach goers received about 10 SED daily during their holidays. For a skin type 2 person (the study recruited mainly Danish subjects who are likely to be skin type 2) this is considered to be about 2.5 to 4.2 MED – enough to cause serious sunburn and to make sunscreen use advisable. Skiers also received 7.5 SED daily, just a little more than outdoor workers with a maximum of 6.4 SED. All situations call for sunscreen use to protect against serious erythema. However, in the laboratory it is common practice to challenge sunscreen with much higher doses − not to predict their behavior at the beach, but to estimate whether a desired claim can be achieved. Typically, doses of 25 MED (62.5 SED) or even 40 MED (100 SED) are used. The endpoint of such radiation experiments could lead to concerns, were they to be performed under realistic use conditions. But to what degree do sunscreens deteriorate when radiated “only” with environmental doses, e.g.: 10 SED? Keeping in mind that while this is a typical value for a sunseeker’s beach holiday, consumers in Japan, concerned about their complexion, would probably be unlikely to expose themselves to more than 0.1 MED (0.5 SED for a skin type
4 person). To answer this question, we performed radiation experiments with commercial sunscreens and exposed them to various doses (see example in Figure 1) up to the 25 MED typical for laboratory challenging. Concentrations of Avobenzone, as a key ingredient of concern, were followed using HPLC. At consumer relevant levels of radiation, it barely decayed and remained stable to 95%, moreover, this represents the value at sunset, after a long beach day. Loss of performance due to photostability therefore poses no concern to consumers due to their relatively low exposure levels compared to artificial challenges in laboratories.
Another concern related to loss of performance is the potential for toxic byproducts to form due to photo-instability. Of course, if a radiation dose is only at environmental exposure levels, a formula’s stability remains rather unchallenged and the amount of photo-products is relatively small. Moreover, for registration approval UV filters have to pass through several photo-toxicity setups to be used in sunscreens, and there is also a requirement to provide skin related safety data under the radiation conditions in which such products will develop. In the case of Avobenzone several large human cohort studies exist which specifically recruited subjects with sunscreen or sun related sensitivity. A recent multi-center study [10] involving 1031 selected subjects utilized photo-patch testing and measured just 1.7% reactions. This result indicates a very low incidence in the random population and we can assume it will be even lower in reality, because in this patch test setup Avobenzone was not stabilized and rather prone to photo-destruction, whereas in sunscreens this process is inhibited by specific UV filters. Such human cohort studies suggest that the impact of potential harmful photo-destruction products is rather limited for Avobenzone.
Photostability and its impact on achieving a very high SPF claim
Should we now stop testing sunscreens artificially in laboratories,using dosages that are almost impossible to get onthis planet? No! For formulators aiming for high protectionSPF claims this is still an essential piece of information. SPFclaims are currently substantiated through in vivo testing inaccordance with standards such as ISO 24444. Under theseconditions, the protective sunscreen film is exposed to veryhigh dosages indeed. For example, to pass for the SPF 50claim, a formulation on human skin is exposed to 50 MED(between 125 and 300 SED depending on the subject’s skintype). The film needs to let just 1/50th of the applied radiationburden through, which then causes a radiation
burdenof 1 MED on skin. By the end of the radiation period, theUV filter composition has been taken to the limit, keepingin mind that such a dosage can hardly be collected on theEarth’s surface [11]. The radiation source is a mix of UVBand UVA light at a ratio of 1:10, UVB being more erythemalthan UVA light by a magnitude of about one, and UVA lightcontributing to about 10% of erythema in the SPF testingsetup. Significant losses on the UVB side would be detrimental,but even though losses on the UVA side are 10 timesless important, they should also be limited, e.g.: throughstabilization or by avoiding unstable UV filter combinations.For example, to achieve high SPF numbers, Avobenzoneshould always be formulated with a triplet quencher such asBis-Ethylhexyloxyphenol methoxyphenyl triazin (BEMT) andnot combined with OMC. Many commercial examples, suchas the one shown in Figure 1, illustrate that by stabilizingAvonbenzone with BEMT it is easy to achieve the 50+ claim.SPF testing in regard to photo-stability is an “integrative”measurement, meaning a photo-unstable sunscreen startswith high protection and ends up lower. One the basis of reaction kinetics one can calculate an quasi stable average of Avobenzone considering the whole period.
Ironically, photo-unstable formulas offer consumers more protectionduring the short period in which they are exposed toradiation, e.g. on the beach, than their labels indicate, asthey need to compensate for the loss in the later phase ofSPF testing. However, that radiation dosage phase is neverreached on Earth as the day of exposure is terminated in theafternoon by sundown.
Photostability in the environment
Sunscreens, and their UV filters in particular, are also a concernin terms of their impact on the environment, especiallymarine fauna and flora. Recently, the Hawaiian governmentbanned two UV filters due to concerns raised in laboratoryexperiments [12] that they may damage corals around theHawaiian islands. Although most recreational sunscreens areformulated water resistant, it cannot be ruled out that a fractionis rinsed off by water. The impact that a material releasedinto the environment has depends on its toxicity, which inturn is species dependent, and on the length and intensity ofexposure. Fortunately, most compounds are degraded by micro-organisms, but the speed of destruction can differ greatly.
Ideally, compounds should be broken down quickly, which is the case if they had been tested to be “readily" biodegradable”. Interestingly, although OMC has this feature it is stillone of the compounds banned in Hawaii. Metabolic destructionby micro-organisms is not the only way by which a compoundcan be eliminated from the environment. Abiotic pathwayscan also destroy molecules, especially with the help ofsun radiation. Although photo-instability presents sunscreenformulators with challenges, the same feature is appreciatedin the environment. We recently conducted exposure experimentswith Avobenzone in quartz cuvettes in water attypical environmental concentrations just below its solubility level and found that even after two days at a window (UVAlight only!) it was broken down by 60-70%, indicating fastelimination from exposed water streams. When reviewingthe literature on water analysis studies of UV filters in lakes,rivers and the sea [13,14] Avobenzone is seldom found, andonly in low concentrations, giving a further indication thata fast abiotic elimination process may indeed take placethrough sun light, at least in layers of water that are close tothe surface. And it is these layers which harbor the speciesa compound may be exposed to. In that sense, photo-instabilitybecomes an interesting design feature in UV filters andcould make a product more environmentally compatible. Ofcourse, sunscreen formulators need solutions to overcomephotostability challenges, as they have with Avobenzoneby combining it with triplet quenchers. But when releasedinto the environment the liaison with stabilizers is broken bydilution enabling deterioration to take place. In this sense,although they were not aware of this when they designedthe molecule, the inventors of Avobenzone built in an environmentalexit switch.
Conclusion
Photostability is a hot topic for sunscreens, however, in some ways its impact is overrated or wrongly interpreted. It plays a significant role in formulating sunscreens, especially for high performance products at SPF 50 and 50+ level. Here, formulators need to be aware of stabilization techniques and problematic combinations they should avoid. For consumers however, if a sunscreen has been assessed at SPF 50 level or higher, it is indeed sufficiently photo-stable, otherwise such a claim would not manifest. Furthermore, with the radiation doses sunseekers at the beach are exposed to, components barely break down. There is no particular reason for concern about losing protection performance after a short period of exposure. When it comes to the environment, photo-degradation by sunlight is a positive feature because it can help eliminate the compound faster than biodegradation through micro-organisms alone. However, a temporary solution during its application in sunscreens and on human skin is required.
Author
Jürgen Vollhardt
DSM Nutritional Products Ltd | Switzerland
References
[1] Lhiaubet-Vallet V, Marin M, Jimenez O, Gorchs O, Trullas C, Miranda MA. Filter- filter interactions. Photostabilization, triplet quenching and reactivity with singlet oxygen. Photochemical & Photobiological Sciences. 2010;9:552-8.
[2] Berset G, Gonzenbach H, Christ R, Martin R, Deflandre A, Mascotto RE, et al. Proposed protocol for determination of photostability Part I: cosmetic UV filters. International Journal of Cosmetic Science. 1996;18:167-77.
[3] Hoeppe P, Oppenrieder A, Erianto C, Koepke P, Reuder J, Seefeldner M, et al. Visualization of UV exposure of the human body based on data from a scanning UV-measuring system. Int J Biometeorol. 2004;49:18-25.
[4] Petersen B, Triguero-Mas M, Maier B, Thieden E, Philipsen PA, Heydenreich J, et al. Sun behaviour and personal UVR exposure among Europeans on short term holidays. Journal of Photochemistry and Photobiology B: Biology. 2015;151:264-9.
[5] Petersen B, Thieden E, Philipsen PA, Heydenreich J, Wulf HC, Young AR. Determinants of personal ultraviolet-radiation exposure doses on a sun holiday. British Journal of Dermatology. 2013;168:1073-9.
[6] Kasper G, Ole Steen M, David Zim S, Brian K, Paul-Anker L, Kristina Sophie I, et al. Solar UV exposure among outdoor workers in Denmark measured with personal UV-B dosimeters: technical and practical feasibility. BioMedical Engineering OnLine. 2017:1.
[7] Moldovan HR, Wittlich M, John SM, Brans R, Tiplica GS, Salavastru C, et al Exposure to solar UV radiation in outdoor construction workers using personal dosimetry. Environmental Research. 2020;181.
[8] Diffey BL, Jansén CT, Urbach F, Wulf HC. The standard erythema dose: a new photobiological concept. Photodermatology, Photoimmunology & Photomedicine. 1997;13:64-6.
[9] Harrison GI, Young AR. Ultraviolet radiation-induced erythema in human skin. Methods. 2002;28:14-9.
[10] Alastair C. Kerr TEMPTS, Taskforce. A European multicentre photopatch test study. British Journal of Dermatology. 2012;166:1002-9.
[11] Williams JD, Maitra P, Atillasoy E, Wu MM, Farberg AS, Rigel DS. SPF 100+ sunscreen is more protective against sunburn than SPF 50+ in actual use: Results of a randomized, double-blind, split-face, natural sunlight exposure clinical trial. J Am Acad Dermatol. 2018;78:902-10.e2.
[12] Downs CA, Kramarsky-Winter E, Segal R, Fauth J, Knutson S, Bronstein O, et al. Toxicopathological Effects of the Sunscreen UV Filter, Oxybenzone (Benzophenone-3), on Coral Planulae and Cultured Primary Cells and Its Environmental Contamination in Hawaii and the U.S. Virgin Islands. Archives of environmental contamination and toxicology. 2016;70:265-88.
[13] Ramos S, Homem V, Alves A, Santos L. Advances in analytical methods and occurrence of organic UV-filters in the environment — A review. Science of The Total Environment. 2015;526:278-311.
[14] Mitchelmore CL, Burns EE, Conway A, Heyes A, Davies IA. A Critical Review of Organic Ultraviolet Filter Exposure, Hazard, and Risk to Corals. Environmental Toxicology and Chemistry. 2021;40:967-88.






