How Can the Right Choice of Packaging Materials Prevent a Loss of Quality in Sun Care Products?
Sun Care products contain active ingredients protect the skin before, during and after sun exposure. However, these active ingredients can lose their effect due to the influence of light and temperature during storage. To prevent this, packaging can be specifically adapted to the requirements of these products in terms of light protection. In addition, the packaging also protects the products from oxidative influences. An adapted oxygen and water vapor barrier can guarantee high product quality over the storage period. Currently, there is a great demand for sustainable packaging. However, these often lead to compromises in terms of barrier properties and product quality. Current research is determining how the product’s requirement for packaging can be reconciled with sustainability. With the help of so-called digital modeling tools, product quality can be predicted over the storage period, thus facilitating sustainable packaging selection. -> back to main page
How sunlight, skin damage and Sun Care products are connected
Sun Care products protect the skin from sunburn, skin cancer and premature aging.
Sunlight has some positive effects on humans. For example, it has a mood-lifting effect by inhibiting melatonin synthesis, can promote blood circulation through infrared radiation and stimulates the skin to produce the essential vitamin D3 (calcitriol).
However, too much sunlight can cause skin damage. The high-energy UV-B radiation in the light spectrum can penetrate into the dermis of the skin. Damage to keratinocytes in the epidermis causes inflammation (sunburn), which reaches its maximum after 12-24 hours. The lowest dose to achieve a perceptible reddening of the skin when irradiated with light of wavelength 297 nm (UV-B radiation) at 21 mJ/cm2 is called the minimum erythema dose (MED). However, DNA damage due to strand breaks, photoadducts, or base dimerization due to the formation of free radicals (reactive oxygen species, ROS) is possible from 60% of MED. This damage can lead to skin cancer. Activation of matrix metallo-proteases by UV-A radiation degrades collagen and elastin in the skin, causing it to lose elasticity. This leads to the formation of wrinkles and a thickening of the stratum corneum and thus to light-induced skin aging. In addition, UV radiation inhibits sebum production and can thus dry out the skin [1]. The human skin has its own UV protection through the
formation of the brown pigment melanin. This pigment is polymerized from the amino acid tyrosine to form eumelanin and pheomelanin. However, it only protects the skin with a sun protection factor (SPF) of 3-10 and lasts for a few months. This protection is not sufficient to spend a long time in the sun in case of sensitive skin types and weather with a higher UV index [2].
Sun Care products with a high sun protection factor (SPF) are therefore ideal for protecting and caring for the skin. Organic or mineral UV filters are contained in the products. Organic UV filters are often derivatives of camphor, salicylic acid or cinnamic acid. They absorb high-energy UV radiation and reemit it as lower-energy, longer-wave radiation. The concentrations used for the individual substances range from 4% (4-methylbenzylidene camphor) to 10% (e.g. homosalates, benzophenone-3, octocriles) [3].
Although UV filters protect well against sunburn by absorbing the light energy, the molecules themselves can also be damaged as a result. While the natural skin pigment melanin converts nearly 100% of UV radiation into heat, organic filters are far less effective. In the case of octyl methoxycinnamate (4-methoxycinnamic acid 2-ethylhexyl ester), the efficiency level is still 80%, while in some others it is less than 50%. The remaining energy leads to chemical degradation of the active ingredients. On the one hand, this can reduce the effectiveness of the product, and on the other hand, it can lead to allergic reactions and hormone-like effects [4,5,6].
Special requirements of Sun Care products for packaging
The absorption of UV radiation takes place not only on the skin, but often already in the packaging. UV rays can penetrate the packaging and damage the UV filters for example in retail lighting on the sales rack, through the window in the bathroom or in direct sunlight on the beach. The high temperatures that develop in the packaging in the process also accelerate the chemical degradation reactions. Therefore, photoprotective barriers are very important for Sun Care products to protect the active ingredients and the consumers.
Oxidation reactions can take place faster under the influence of light than in the dark. In particular, autoxidative processes can be initialized by high-energy light such as UV radiation. The exclusion of UV light, e.g. by using effective UV filters in transparent packaging, can provide product protection and increase quality retention.
Many plastics already have reduced light transmission in the UV-C and UV-B range (see Figure 1). Transmittance is a measure of the light transmission of a material at a given wavelength. It can be used to compare the ability of different plastics to absorb UV radiation. For example, polymethyl methacrylate (PMMA, acrylic glass) absorbs UV radiation completely up to 235 nm, while polyethylene terephthalate (PET) still shows complete absorption at 310 nm [7].
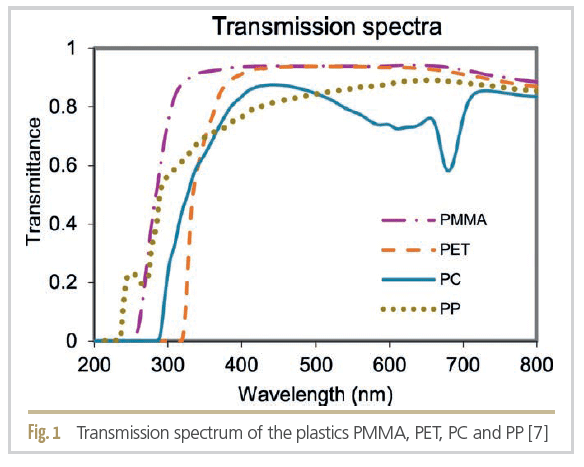
PET and polypropylene (PP) are commonly used for bottles and tubes of cosmetic products. Acrylic glass (PMMA) is often used for transparent packaging such as jars, powder compacts and lipgloss tubes. However, oxidation reactions and active ingredient degradation can occur because these materials are transparent to most of the UV-A radiation between 280 to 400 nm.
Mineral or organic UV absorbers, similar to sunscreen products, can also be incorporated into packaging to increase light protection. Inorganic substances are used, for example, as nanoparticles of titanium dioxide, zinc oxide and iron oxide. Benzotriazole or benzophenone absorbers, for example, can be used as organic additives for plastics. Since sunlight primarily emits UV radiation from 290 nm to 400 nm, UV filters must absorb the radiation almost completely across the entire wavelength spectrum. Although Sun Care products are preferably purchased in the summer, they are available in stores throughout the year. To ensure effective product protection even under retail lighting, UV filters for plastic packaging should maximize absorption of UV light, especially at 365 nm, the main UV emission peak of fluorescent lamps. Although these agents can be incorporated into the plastic, this often leads to disadvantages during recycling. Applying a coating layer containing UV filters to the surface of the packaging can ensure product protection and sustainability [8,9].
To determine the effect of different UV filter coatings, UV filter active ingredients were incorporated into a coating and doctored onto a plastic film. A barrier film was used for the coating (PET 12 μm with 5 μm EVOH). Polyvinyl lactate Vinnapas 4fs (Wacker Specialities) was used as coating base. The following procedure was used to prepare the coating solutions: 5 g of coating base was dissolved in 50 ml of solvent (ethyl acetate). The UV filter substances were added to the varnish solution in the amounts of 15% w/w - based on the dry masses - and also dissolved. The film was coated in DIN A3 format on the Coating Unit CUF5 coating machine from Sumet-Messtechnik at the Fraunhofer IVV. The coatings with the different UV filter substances were applied in defined layer thicknesses to the barrier film with a blade and then dried in the coating machine [10].
The light transmission was investigated spectroscopically by means of transmission measurements. The materials were placed in the beam path of a spectrophotometer with integrating sphere (Ulbricht sphere) and examined in the wavelength range of 200 - 800 nm at intervals of 1 nm. With the aid of the integrating sphere, not only the directly transmitted radiation component but also the scattered light was focused and detected.
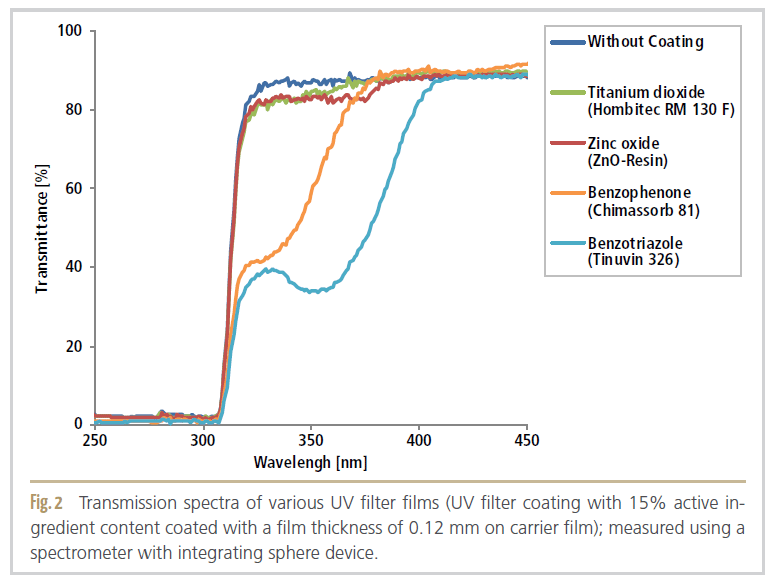
Figure 2 shows that Tinuvin 326 exhibited the best UV filter effect compared to the other UV filter substances and absorbed a large proportion of the light up to 400 nm over a wide wavelength range.
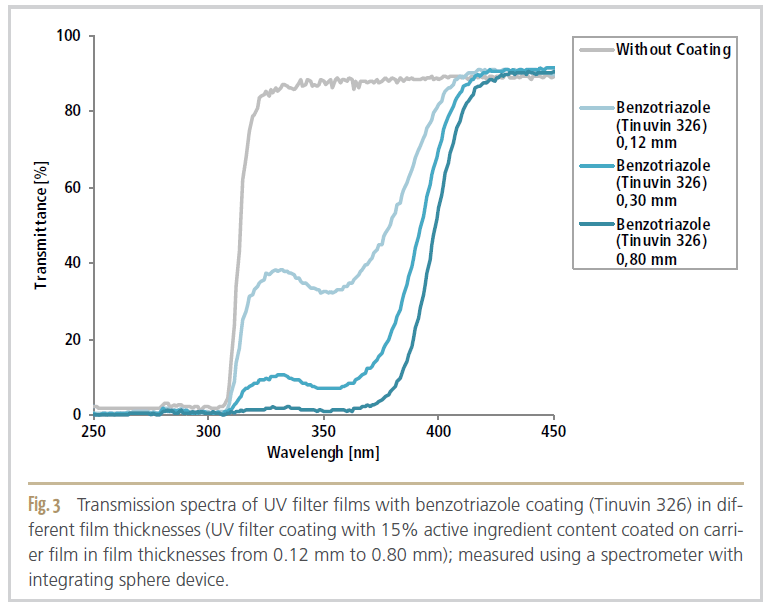
Subsequently, the resist film thickness was increased in several steps and thus more UV filters were applied per area in order to increase the absorption of the UV filters. It can be seen, that with increasing film thickness, the light transmission in the UV range decreased (Figure 3).
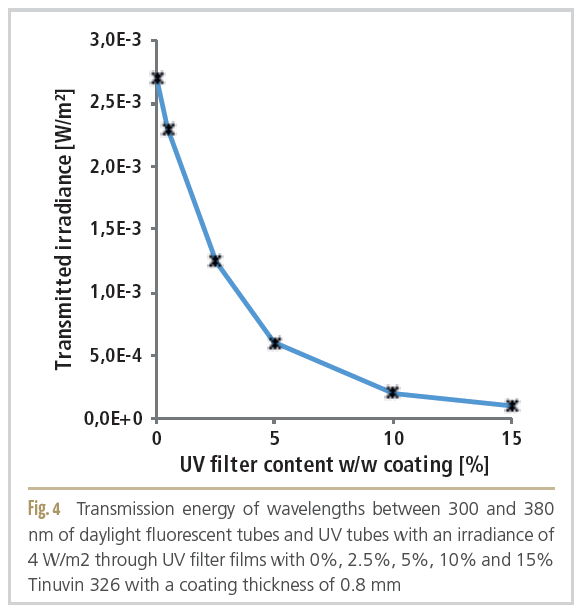
To analyze the light transmission spectrum under trade illumination, a DAD sensor was inserted into the package and the difference spectrum between the trade illumination inside and outside the package in the range of 200 - 800 nm was evaluated (Figure 4). The analysis of the light transmission spectrum of the films with the benzotriazole varnish coating
(Tinuvin 326) in concentrations from 0 to 15% was carried out with exposure to daylight fluorescent tubes with an irradiance of 4 W/m2 and an additional UV tube with a film thickness of 0.8 mm. Compared to the film without coating, the UV filter film transmitted only 3.7% of the irradiance [W/m2] between 300 and 380 nm.
The light barrier in packaging is an important aspect for product protection, as it protects the product from photooxidation and light-induced active ingredient degradation and can thus extend shelf life.
Oxygen and water vapor permeability of cosmetic packaging
In addition to the light barrier, oxygen and water vapor barriers are also essential for maintaining the quality of cosmetic products [11,12].
A suitable oxygen barrier can protect products from oxidation. To preserve the moisture and regenerative capacity of the skin, unsaturated fatty acids, such as ã-linolenic acid [13,14] and antioxidants, such as carotinoids [15] are essential. These can neutralize free radicals and build up the skin’s protective barrier. However, atmospheric oxygen can damage the sensitive ingredients, which in the case of unsaturated fatty acids can lead to rancid, fishy, bitter or pungent off-flavors. Antioxidants lose their regenerative effect through oxidation. The simultaneous presence of light leads to photooxidation, which significantly accelerates the oxidation rate. Photosensitizing substances can
accelerate this reaction furthermore, for example chlorophyll. Due to its lipophilic properties, chlorophyll can pass into the cosmetic ingredients during oil extraction from plants or during the production of plant extracts from herbs. Many other factors, such as the large interface in emulsions, increase the oxidative susceptibility of the cosmetic product [16,17].
Water vapor entering an anhydrous product through the packaging can lead to hydrolysis of the triglycerides to free fatty acids. Free fatty acids exhibit a pungent, rancid, musty or soapy aroma. Diffusion of water vapor from water-containing products through the packaging into the atmosphere can lead to weight loss, which may result in non-compliance with the weight or volume declaration on the product or in instability of emulsions due to a change in the ratio of water to oil phase.
By providing an adequate oxygen and water vapor barrier, the ingredients and formulation can be protected with the help of the packaging.
Typical barrier materials for tubes, bottles and jars contain EVOH interlayers. In addition, ultrathin metallizations, ceramic layers of ultrathin silicon oxide, and barrier coatings with nanomaterials are also possible. The high density of the polymer, low mobility of the molecular network and intermolecular interactions, such as hydrogen bonding, reduce gas movement and provide a high barrier. It should be noted that different barrier materials may also have different permeabilities for oxygen, water vapor, or even aromatic substances. Due to the different affinity of the gas molecules to the polymer network, they can dissolve in the packaging in different strengths and migrate through it.
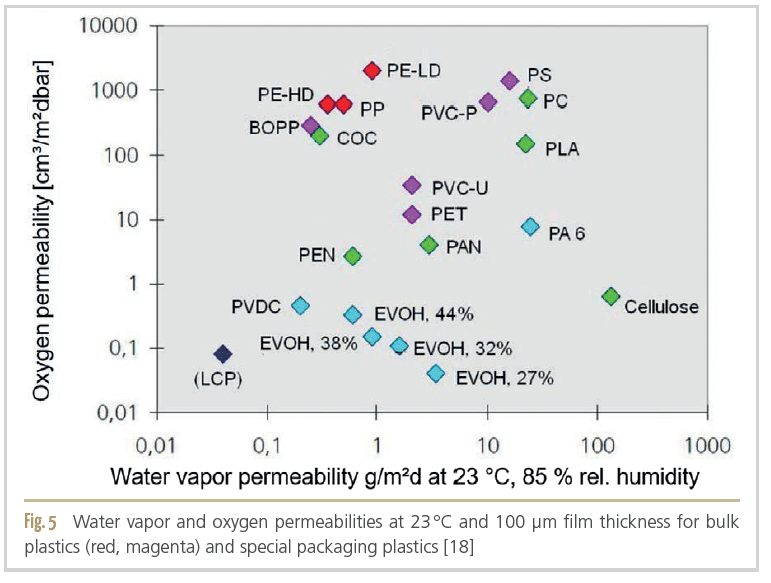
Barrier properties in terms of oxygen, carbon dioxide and water vapor permeation are important criteria for product protection, as they can protect the product from loss of inert gas or oxygen penetration and extend microbiological and oxidative shelf life. Plastics commonly used in packaging are shown in Figure 5 with their respective normalised water and oxygen permeabilities. Depending on the sensitivity of the packaged products, the packaging material should be selected to provide the products with the protection required in each case [18].
The investigation of the permeability of flat as well as formed packaging materials can be carried out in various ways. Oxygen permeability measurements are often carried out in accordance with DIN 53 380-3 using an oxygen-specific carrier gas method. Here, the amount of oxygen that has passed through the sample is detected by an electrochemical sensor. The measurements are typically carried out at 23 °C and 50% relative humidity.
To determine the CO2 permeability of the packages, the gas composition in the packages is tested after storage in a 100% CO2 atmosphere. Since the packages are filled with air (21% O2 and 79% N2) before storage, CO2 permeates into the package due to the partial pressure gradient, and N2 and O2 permeate out of the package in the opposite direction. With the aid of mathematical calculations (gas permeation kinetics), the gas permeabilities can be calculated from the measured values. The measurements are taken at 23 °C and 0% relative humidity. The water vapor permeability test is carried out in accordance with DIN 53 122-1 (gravimetric method) at a temperature of 23 °C and a relative humidity gradient of 85% to 0%.
Product shelf life when using sustainable packaging
To protect sensitive products from oxygen, they are often packaged in multilayer film packaging under vacuum or inert gas. These multilayer plastics are made of at least two materials and do offer higher barrier properties against oxygen, water vapor, light, organic substances and other environmental influences. However, multilayer packaging made of different materials often also has poorer recyclability.
With greater environmental awareness, recyclable, biodegradable and sustainable packaging is being demanded by consumers. Glass containers offer good recyclability, but due to their weight they increase CO2 emissions during transport and pose a risk to the consumer due to possible breakage. Therefore, plastic remains the material of choice.
However, recyclable monomaterials such as PE, PP and PET offer lower barrier properties than multilayer packaging. The product’s quality can be compromised by the influence of light and oxygen. Therefore, special consideration must be given to the oxidation sensitivity of the bulk, the light and gas permeability of the packaging
material, and the wall thickness of the packaging when selecting a packaging material for the desired shelf life. Further research is needed to develop suitable packaging that fully meets the requirements of sustainability, recyclability and product quality preservation. In order to constantly gain new insights, scientists at the Fraunhofer Institute IVV are constantly ongoing research in the areas of process engineering, packaging, product impact, recycling and product shelf life. Digital methods, such as chemical/physical shelf life models (so-called “shelf life modeling”), can be accelerate development cycles. Thereby, the shelf life of products can be modeled as a function of the packaging and storage conditions.
In summary, the wrong choice of packaging materials can deteriorate product quality. If light protection and oxygen barrier are insufficient, chemical degradation and (photo-) oxidation of active ingredients may occur. Inadequate water vapor barrier may result in product weight and stability change. However, proper selection of packaging materials can contribute to longer shelf life and improved quality of the product.
Authors
Arielle Springer*, Dr. Matthias Reinelt, Marius Jesdinszki, Joachim Wunderlich
Fraunhofer-Institut für Verfahrenstechnik und Verpackung IVV |
Giggenhauser Str. 35 | 85354 Freising | Germany | www.ivv.fraunhofer.de
*Corresponding author:
Arielle Springer | Research Associate |
Mail: [email protected] | Tel.: +49 8161 491 470 | Mobile:+49 1716 411 383
Bibliography
[1] Lohan S. B. et al. (2016). Free radicals induced by sunlight in different spectral regions - in vivo versus ex vivo study. Exp Dermatol;25(5):380-5
[2] Baran R. & Maibach H. I. (2017). Textbook of cosmetic dermatology, Boca Ranton: Taylor & Francis Group, LLC
[3] Verordnung (EG) Nr. 1223/2009 des Europaischen Parlaments und des Rates vom 30. November 2009 uber kosmetische Mittel; http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:342:0059:0209:DE:PDF
[4] Stebut E. (2015). Reisedermatosen. Springer Verlag S. 303
[5] Shaw T. et al. (2010). True photoallergy to sunscreens is rare despite popular belief. Dermatitis;21(4):185-98
[6] Schlumpf M. et al. (2001). In vitro and in vivo estrogenicity of UV screens. Environ Health Perspect;109:239-244
[7] García-Gil A., Pablos C., García-Muñoz R. A., McGuigan K. G., Marugán J. (2020). Material selection and prediction of solar irradiance in plastic devices for application of solar water disinfection (SODIS) to inactivate viruses, bacteria and protozoa, Science of The Total Environment, Vol. 730
[8] Danzl W. & Ziegleder G. (2007). UV protection for packed foods. New Food 10, 37–42
[9] Danzl W. & Ziegleder G. (2005). UV-Filter in Lebensmittelverpackungen. Chancen und Grenzen fur den Produktschutz. Verpackungs-Rundsch. 56, 63–66
[10] Final report IGF research project number 17608 N (2015): Development of a MAP packaging concept for fresh meat using UV filter films and optimized modified gas atmosphere.
[11] Piringer O.-G. & Baner A. L. (2008). Plastic packaging: Interactions with food and pharma-ceuticals. 2nd ed. Aufl. Wiley-VCH, Weinheim Chichester
[12] Robertson G. L. (2012). Food Packaging: Principles and Practice, Third Edition. 3rd ed. Aufl. CRC press, Hoboken
[13] Ferreira M. J., Fiadeiro T., Silva M. et al. (1998). Topical ã-linolenic acid therapy in atopic dermatitis. Allergo J 7: 213–216
[14] Van Gool C., Zeegers M., Thijs C. (2004). Oral essential fatty acid supplementation in atopic dermatitis—a meta-analysis of placebo-controlled trials. British Journal of Dermatology 150: 728-740
[15] Souza C et al. (2017). Radical-Scavenging Activity of a Sunscreen Enriched by Antioxidants Providing Protection in the Whole Solar Spectral Range. Skin Pharmacol Physiol;30:81-89
[16] Schieberle P., Haslbeck F., Laskawy G. & Grosch W. (1984): Comparison of sensitizers in the photooxidation of unsaturated fatty acids and their methyl esters. Zeitschrift für Lebensmittel-Untersuchung und Forschung, 179, 2: 93–98
[17] McClements D. J., Decker E.A. (2000). Lipid Oxidation in Oil-in-Water Emulsions: Impact of Molecular Environment on Chemical Reactions in Heterogeneous Food Systems. Journal of Food Science, Vol. 65 (8)
[18] Langowski H.-C. (2008): Permeation of Gases and Condensable Substances Through Monolayer and Multilayer Structures. In: Piringer O.-G. & Baner, A. L. (2008) Plastic packaging, 297–347. Wiley-VCH, Weinheim Chichester






